PRODUCTS
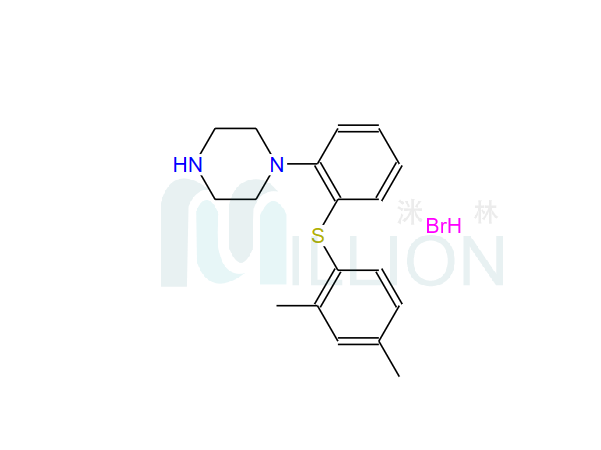
Product name: Votioxtine hydrobromide
Cas NO.: 960203-27-4
M.F: C18H23BrN2S
M.W.: 379.36
Capacity: 100KG/M
Usage: Central nervous system
■ Description
General: Votixetin hydrobromide is considered to be a new multi-model antidepressant drug. In vitro studies have shown that it can antagonize 5-HT3, 5-HT7 and 5-HT1D receptors, activate 5-HT1A receptors, and partially activate 5-HT1A receptors. HT1B receptor and inhibition of 5-HT transport. It is a slightly yellowish white powder that is slightly soluble in water. Votexeting is an antidepressant with multiple mechanisms of action, acting not only on 5-HT transporters but also on a variety of 5-HT receptors. This drug has high affinity to 5-HT transporters, which can bind various 5-HT receptors and can exert multiple pharmacological effects by binding to the corresponding transporters and receptors.
Product introduce:Vrtioxetin hydrobromide is a drug used for the treatment of depression and anxiety. Vrtexetine hydrobromide is considered as a new multi-model antidepressant. In vitro studies show that it can antagonize 5-HT3, 5-HT7 and 5-HT1D receptors, activate 5-HT1A receptors, partially activate 5-HT1B receptors and inhibit 5-HT transport.
Function: It is an antidepressant. It is used to treat anxiety and depression. Votisidine hydrobromide is a yellowish white powder, which is slightly soluble in water.
Origin:Votioxetne hydrobromide is a new diaryl thioalkylamine antidepressant jointly developed by Takeda, Japan and Lingbei, Denmark, which is used for the treatment of depression and anxiety.In September, 2013, it was approved by the FDA of the United States to be listed as Brintelix, which was used for the treatment of major depression in adults. In October of the same year, the application for marketing permission (MAA) of Voetixtine received positive opinions from the Committee of Medical Products for Human Use (CHMP) of the European Drug Administration (EMA), and CHMP suggested to approve Brintelix for the treatment of major depression (MDD) in adults.In December, 2013, the European Commission of EMA granted the marketing right of Voetixtin in the whole European Union. There are four listing specifications of Voetixetie: 5mg, 10mg, 15mg and 20mg. At present, the application for listing of Vortextine Hydrobromide has been submitted in many countries, but it has not been listed in China.
Vootixetin has high affinity with 5-HT receptor. Compared with duloxeting, Vootixeting can act on 5-HT3, 5-HT1A, 5-HT7, 5-HT1D and 5-HT1B receptors with high selectivity, while regulating mood, the dosage is small, the once-a-day administration scheme is simple, the patient's compliance is improved, and there are few drug interactions, high selectivity and few side effects. As a new antidepressant, Votisidine is considered to be the most successful drug in unipolar affective disorder research.
■ Message
Related Products
Leave A Message
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.