PRODUCTS
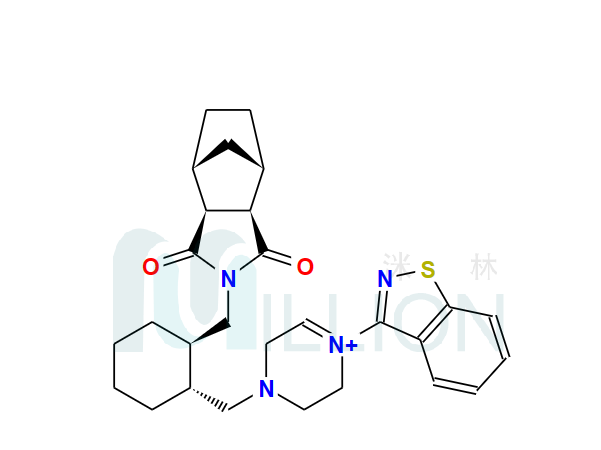
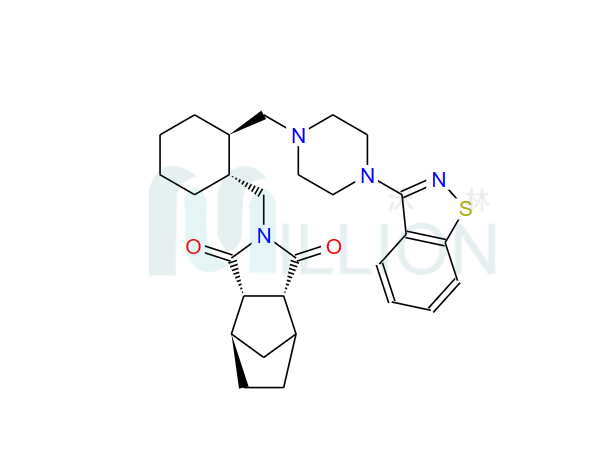
Product name: Lurasi-done
Cas NO.: 367514-87-2
M.F: C28H36N4O2S
M.W.: 492.68
Capacity: 200KG/M
Usage: Central nervous system
■ Description
General: cas 367514-87-2 lurasidon base is an atypical antipsychotic, and the exact mechanism for treating schizophrenia remains as unclear as other atypical antipsychotics and may be related to the antagonism of dopamine D2 and 5 serotonin 2A (5-HT 2 A) receptors. It is used for the treatment of schizophrenia, and some studies have reported that lur asidone can improve cognitive function. The hcl salt lurasidon is API also large stock.
Product introduce:Lur-asidone is suitable for the treatment of schizophrenia. Lurasdone is an antagonist of dopamine D2 and 5-HT7, with IC50 values of 1.68 and 0.495 nM, respectively and is also a partial agonist of 5-HT1A receptor with IC50 value of 6.75 nM.
Function:Lurasidon is used to treat mental illness. Many clinical trials show that it can improve psychotic symptoms, emotional symptoms and cognitive symptoms of schizophrenia patients, and has the safety advantages of little influence on glucose and lipid metabolism indexes and prolactin.
Origin:Lurasidon is an atypical antipsychotic drug developed by Dainippon Sumitomo Pharmaceutical Company of Japan. On October 28th, 2010, the US Food and Drug Administration (FDA) approved its marketing under the trade name for the treatment of schizophrenia.
■ Message
Related Products
Leave A Message
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.